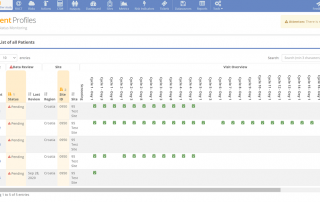
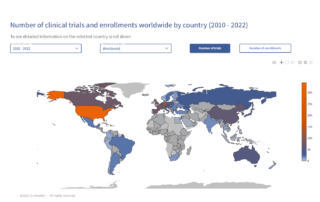
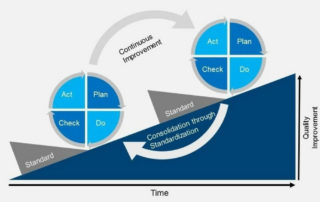
Revolutionizing Clinical Data Management in the US: A Look at the Past, Present, and Future
Discover how clinical data management in the US has transformed from data collection to a multi-faceted endeavor, driving breakthroughs in biomedical research, diagnostics, drug development, and vaccines. Learn from leading experts, including our Chief Scientific Officer, about the latest advancements and future prospects in this groundbreaking publication.