Risks of IMP Management Under Standard vs. Pandemic Conditions
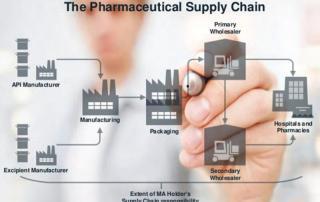
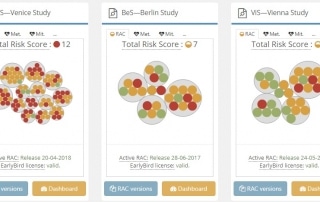
The COVID-19 pandemic has accelerated alternative distribution strategy adoption to facilitate “new normal” decentralized and hybrid trial concepts. Capturing the IMP supply chain risks associated with these more flexible distribution approaches requires synergy among people, processes, and technology. Former Senior Project Manager at GKM, Anke Mueller, shares with you how pandemic-induced supply chain risk data knitted together in a single RAC tool enabled her study team to pivot quickly and continue their studies in a timely fashion.