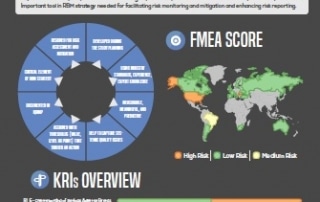
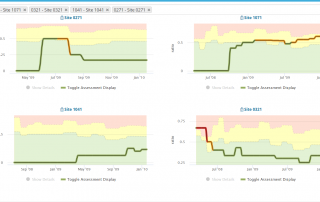
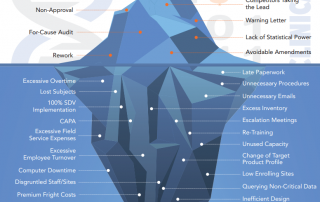
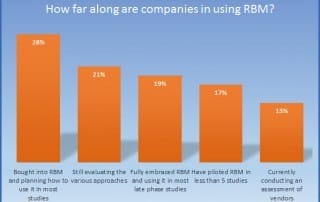
Centralized Monitoring: a Greater Advantage to a Broader Range of Trials
Regulatory bodies are unanimous, today's clinical research needs Centralized Monitoring. We summarized Centralized Monitoring the infographic way, download your free copy here...