Process Flowcharts for Adaptive Monitoring
The Complete Series of Adaptive Monitoring Process Flowcharts
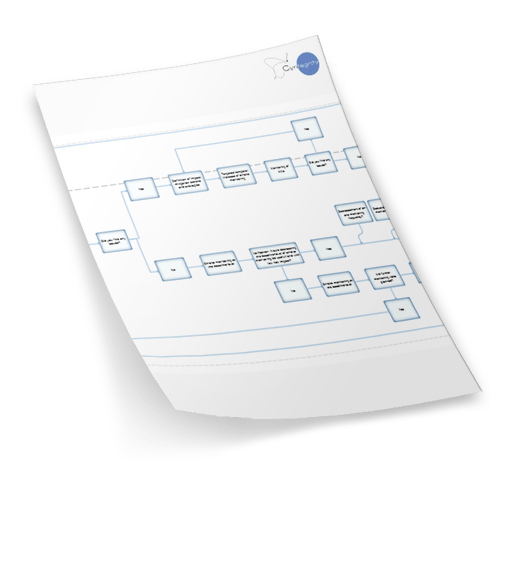
Overview
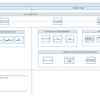
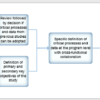
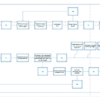
Adaptive Monitoring (AM) process flowcharts will greatly improve your clinical monitoring process. This series of process flowcharts results from Cyntegrity’s long years of experience in implementing AM strategies in diverse clinical settings.
Risk-based Quality Management integrates the AM process because it addresses all aspects of quality risk management. Thus, the concept incorporates people, process, and technology yielding the greatest impact on quality, effectiveness, and savings.
This series of process flowcharts consists of the following chapters:
- AM 1 – Adaptive Monitoring Process Overview
- AM 2 – Identification of Critical Processes and Data
- AM 3 – Study Quality Control
- AM 4 – Risk Identification and Assessment
- AM 5 – Risk Detection and Issue-Management
- AM 6 – Adaptive RBM
- AM 7 – Study Performance Control and Central Monitoring
- AM 8 – Risk Review and Lessons Learned
Read also: Adaptive Monitoring – Anticipating The Human Factor
The process flowcharts (pdf and editable MS Visio file format) can be downloaded immediately after the successful completion of your payment. An invoice will be automatically sent to your email address.
Interested in other Cyntegrity tools that provide clinical study risk management and optimization strategies?
Here at Cyntegrity, our mission is to help pharmaceutical companies stay GCP compliant, improve patient safety, make a clinical study more predictable and optimize monitoring resources. Because we stay true to out mission, our tools and resources are built to reflect it. Make use of the resources and tools our data experts have perfected through years of clinical data research. Check how you can optimize your clinical trial management here.
Pricing
For You
€299,00
- 2 year access
- 100% online
- Unique additional tools
*Courses are limited to 1 item per person. For purchasing for a team, please get in touch.