From EMA Findings to ICH E6(R3): QbD and RBQM in Practice

How EMA inspection findings translate into ICH E6(R3) expectations, and why Quality by Design and RBQM must operate together in clinical trials.
Risk-based quality management in 2026

Risk-based quality management in 2026: how ICH E6(R3) defines RBQM, where practice diverges, and what regulators now expect in clinical trials.
FDA AI Guidance 2025 Interactive Visualizer
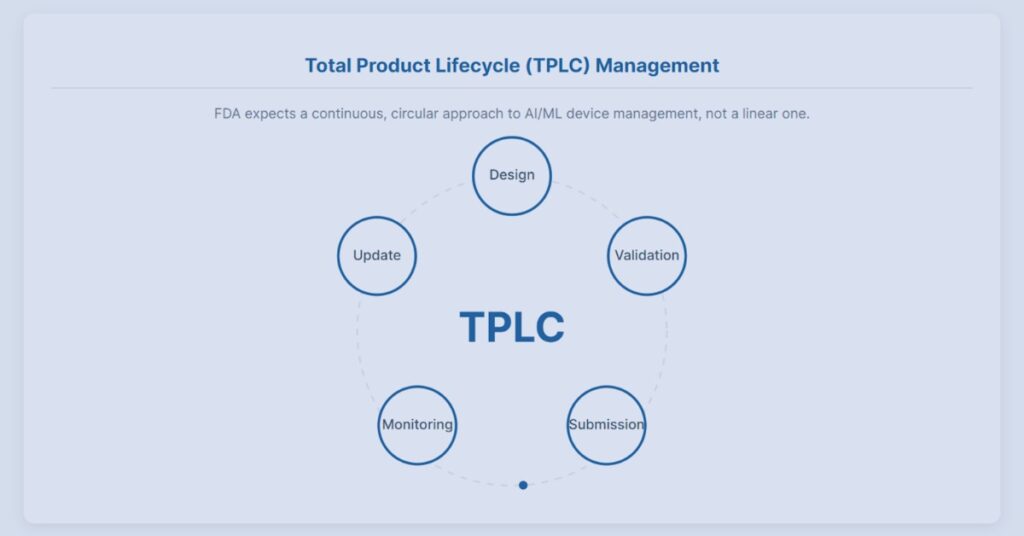
Explore how FDA’s 2025 AI guidance shapes lifecycle oversight for AI-enabled medical software through interactive regulatory scenarios.
ICH E6(R3) Interactive Visualizer: GCP Explained Visually
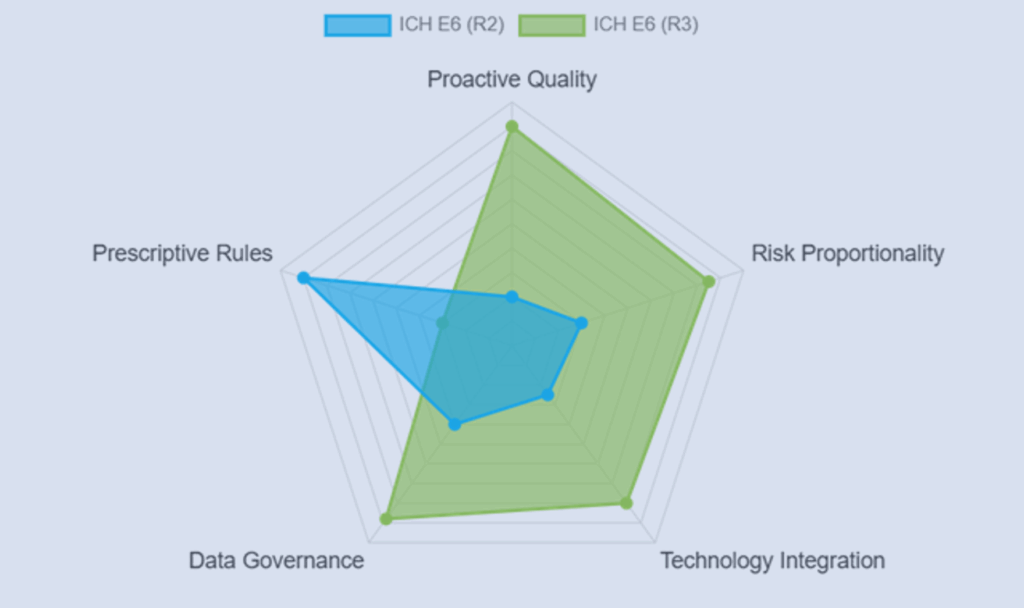
Explore the evolution of Good Clinical Practice with an interactive ICH E6(R3) visualizer showing ethics, risk-based oversight, and modern GCP design.
EMA Clinical Trials Map Visualizer Explained
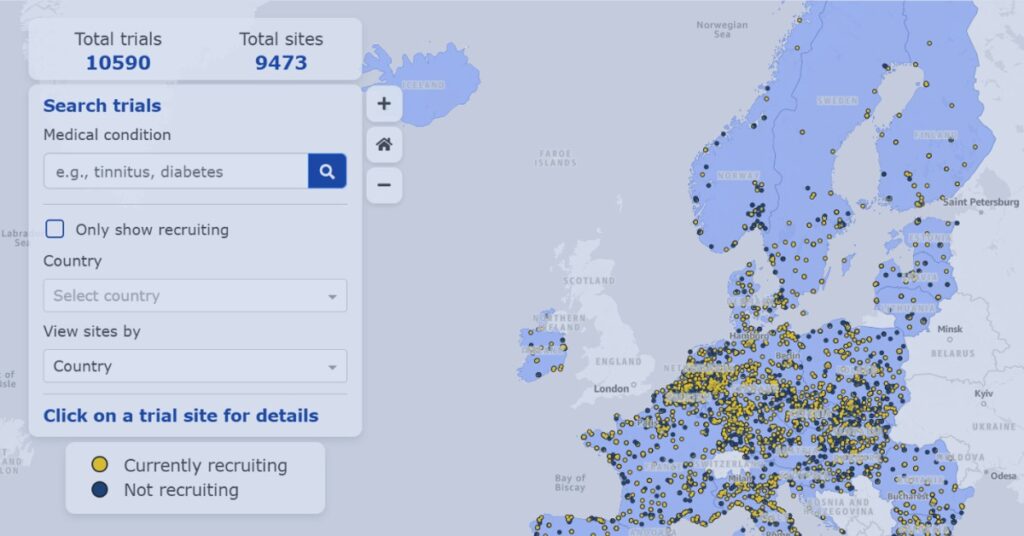
Explore how the EMA Clinical Trials Map visualizes trial activity across Europe to support recruitment planning and site strategy decisions.
MyRBQM® Portal Onboarding Plan

Download the MyRBQM® Portal Onboarding Plan — a realistic 3–6-month roll-out blueprint covering pilots, integrations, governance, validation, and hypercare. Adaptable to in-house, hybrid, and outsourced delivery models.
Phase III Oncology: Targeted Risk-Based QA Case Study

How ADAMAS used AI-augmented RBQM analytics to strengthen oversight, focus QA effort, and improve inspection readiness in a global Phase III oncology study.
Patient Profiles: Understand the Person Behind the Data

Learn how modern medical monitoring uses contextual patient profiles and AI-supported oversight to detect risk sooner and protect patient safety.
Drug Interaction Visualizer: Explore Drug–Drug Safety Profiles

Explore drug–drug interactions using FDA adverse event data. Search, compare, and visualize potential risks when combining commonly prescribed medications.
The Real Cost of Trial Delays & How RBQM Reduces Risk

Clinical trial delays are costly. Learn updated cost estimates and how RBQM + QbD make studies more predictable, efficient, and patient-focused.







