When Conflict-of-Interest is a Hidden Factor of Potential Bias
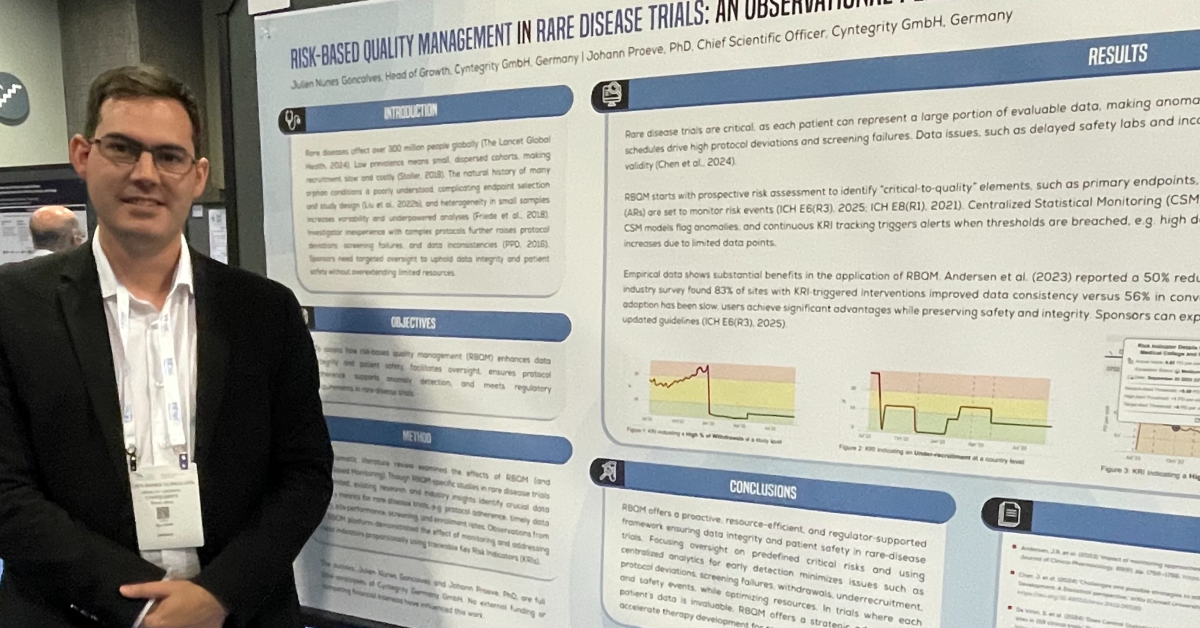
Some of the major challenges (risks!) in clinical trial conduct include delays in data entry, misconduct at the site, degradation of data quality (due to lost-to-follow-up cases/Informed Consent withdrawn cases), etc. Risk Based Quality Management has evolved through the use of data in order to mitigate these risks. For e.g. using audit trails from the EDC systems to check for data integrity, and by establishing timelines of important events. The following case study illustrates how the MyRBQM® system helped solve these problems using the principles of Risk Based Quality Management.
CASE STUDY I: Proper use of Permissible Clarifications
As data integrity is one of the cornerstones of clinical trials, data entry should always be performed by the site personnel. This is mainly done to ‘avoid bias‘. Since the Sponsor has a conflict of interest in the trial’s progress, sponsor company users (e.g. Monitors) may exceptionally make ‘type 1 error corrections’ or ‘permissible clarifications’, not mass data entry.
What went wrong?
Instead of site personnel, the sponsor company monitor entered the majority of the data of two Japanese sites into the EDC system using his user ID for ‘permissible clarification’ or ‘type 1 error correction’.
How was it detected?
The MyRBQM® Portal had been set up such that the entries done by the various parties involved in the study and having access to the EDC system had been counted. It used the audit trail of the EDC system and checked for the sponsor company users entering or modifying data.

How was this risk addressed?
Through the implementation of a Key Risk Indicator (KRI) for User-ID from sponsor company staff and the use of it for data entry. An alert was triggered when the threshold, i.e. in case of more than 0.5% usage of the user-ID for data entry, was reached.
Why was the MyRBQM® Portal used?
- Non-adherence was spotted proactively, instead of retrospectively
- So that misconduct could be mitigated on time using a specific KRI
Download: CASE STUDIES | How the MyRBQM® Portal Optimizes Clinical Trials

The relationship between information and risk/opportunity has changed. Clinical trials generate more searchable information than they used to. Integrated, time stamped audit trails automatically track and record what study teams and site staff do, who they communicate and collaborate with and at what point in time; the technology they use also captures subtler user information, such as how well individual users adhere to the protocol, how quickly they enter the data and how fast they respond to queries.
As more clinical recording systems become interconnected, so the amount of information clinical trials inadvertently create will continue to grow. Risk-based Quality Management has evolved through the use of data in order to mitigate risks and provide optimization opportunities.