Patient Safety in Clinical Trials
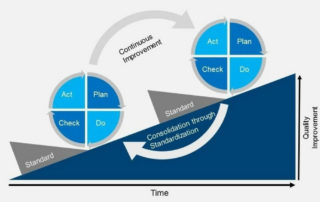
What is the future of patient safety in clinical trials? The life sciences industry is experiencing an evolution. Technology is forcing digital transformation and is rapidly changing the way that companies conduct research, test treatments, and evaluate data. To continue this progress, industry experts [...]