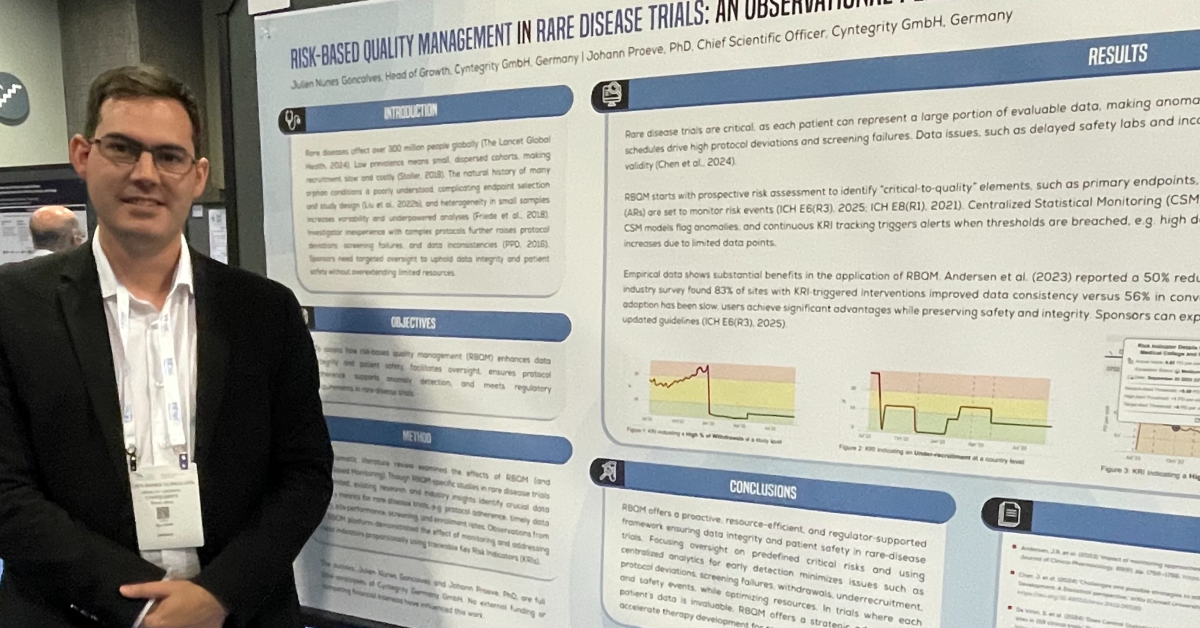
Challenges Associated with Clinical Research in Heart Failure
The American College of Cardiology (ACC) and the American Heart Association (AHA) have devised a classification system for heart failure. It categorizes heart failure based on how the disease progresses in most people. Our Chief Scientific Officer, Dr Johann Proeve, explains the challenges associated with the conduct of clinical studies in the various heart failure stages.
Heart Failure (HF) is a complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood. [ACC]
Stage A – at high risk but no structural disorder
Heart failure is one of the most common cardiovascular diseases with many root causes, such as hypertension, diabetes, obesity, atherosclerotic disease, or due to hereditary reasons. There are many stages of the disease starting without any structural signs or without any symptoms of heart failure. Therapy in these cases focuses on a healthier lifestyle, use of statins or the use of ACE inhibitors. Clinical studies usually do not start at this stage A – a stage where hardly any signs or symptoms of heart failure can be detected.
Stage B – a structural disorder without symptoms
Clinical studies start at stage B of the disease, where structural changes can be observed but no symptoms of heart failure can be observed yet and patients are at risk to develop heart failure. These patients suffered from a myocardial infarction, an asymptomatic valvular disease or in general a low ejection fraction of the left ventricle. These patients are being treated with beta blockers, ACE inhibitors or worst case have to undergo valvular surgery.
Clinical studies may be initiated using drugs to prevent the structural changes not to develop further or the patients moving into a stage with heart failure symptoms. The challenges in such studies are the long-term character of the studies looking for those patients developing heart failure symptoms versus those that do not. Patients may not necessarily strictly adhere to the treatment – standard or study drug treatment – since they do not (yet) suffer from the heart failure symptoms. Reliable study outcomes however, depend on a sound protocol, sites and patients being cooperative.
Stage C – past or current symptoms associated with structural HF
Heart failure studies in stage C get even more complicated since one has to differentiate between those patients with heart failure symptoms and structural changes of the heart with preserved ejection fraction (HFpEF) versus those with reduced ejection fraction (HFrEF). Also, the treatment objectives in these two sub-populations are different, with the HFpEF group objective to improve quality of life and keeping the symptoms under control while preventing hospitalizations and mortality, and in the HFrEF group attempting to achieve the same while also ensuring a sound patient education. There are many different treatment options available, however, these must be adjusted to each and every patient situation.
Again, the clinical trials in this group are complicated and require a lot of treatment adjustments for heart failure but also of the underlying diseases as described above. The trials are usually long-term outcome trials with many patients and also a lot of dosage adjustments requiring a close monitoring of the studies.
Stage D – end-stage disease requiring specialized treatment strategies
Finally, in stage D of the disease, the patients suffer from refractory heart failure symptoms, mainly already at rest and not only during exercise, and frequently require a hospital admission despite a whole set of medications for treatment of the heart failure. The objective of any treatment at this stage is to reduce the hospital admissions, improve the quality of life (as far as that is still possible) and work with the patients on their end of life objectives.
From a clinical drug development point of view hardly any pharmaceutical company will initially run clinical studies in this patient population. Only after having successfully demonstrated that the new drug works in any of the other stages of heart failure, studies may also be initiated in stage D heart failure patients. These studies also need to be carefully planned and supervised, preferably using a data monitoring committee. Clear cut stopping rules need to be put in place in order to ensure that the patients are not harmed.
All of the above shows that running clinical studies in the heart failure population is complex and requires a lot of planning and a sound oversight on what happens in an ongoing trial. Risk based quality management and the indication focused Heart Failure RACT can help ensuring patient safety on one hand and being on top of the study on the other hand.
Read also: MyRBQM® RACT – Indication Focused RACT Library
Try the new Heart Failure RACT catalog with MyRBQM® Portal’s free 30-day user license: https://cyntegrity.com/product/myrbqm-portal-free/