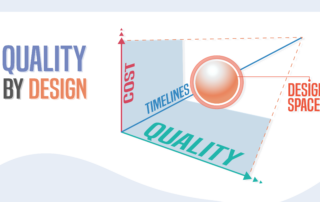
RBQM is laser-focused. Risk-based Quality Management (RBQM) considers each research program holistically, identifies areas of increased risk, and uses that information as the basis for a customized monitoring program. RBQM is not “reduced” monitoring, it is strategic monitoring based on technologically enabled, risk-based algorithms that focus monitoring resources on the locations and activities where they are most needed.
RBQM isn’t static. If risk levels increase at any phase or stage of a study, monitoring can quickly be intensified.
The power of RBQM lies in risk identification and strategic vigilance.