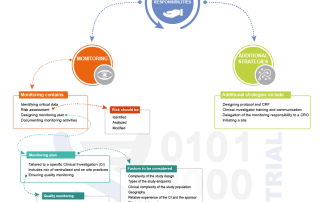
The Emergence of a “Risk Monitor”: Preparing for the future.
Pharmaceutical companies and contract research organizations (CROs) are increasingly trying to leverage technology to optimize risk-based monitoring. While technology is a critical component, roles also need to be looked at again. Recently we interviewed Dr. Nimita Limaye, an expert in risk-based monitoring, about the future [...]