Do you want to try RBM on practice? Still confused by many offers and companies? Do you want to decide yourself based on objective evidence?
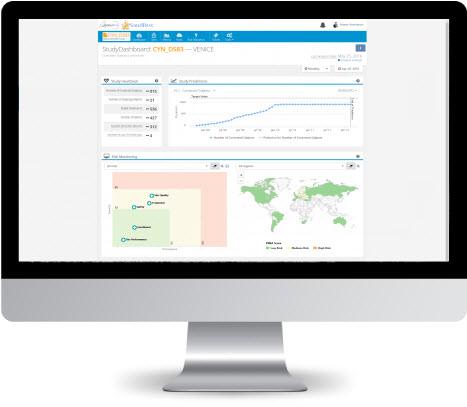
Apply and test our newest RBM demo environment. Explore the holistic RBM process and get a feeling how technology could support your team. Test the latest features of EarlyBird 4.0 with real clinical trials.







Leave A Comment
You must be logged in to post a comment.