The majority of companies are delaying the RBM adoption. Why? The reason lies now in the nature of innovation adoption.
The main hesitations of the pragmatists to apply RBM nowadays are connected with:
- the general complexity of the approach
- the perceived risk of audit findings
- the discomfort with required process change
A recent survey during a webinar of an RBM technology provider showed the current RBM adoption status:

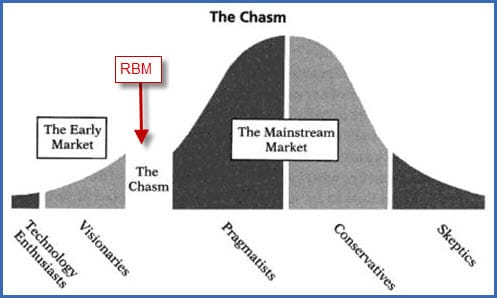
Almost half of the companies (28% + 21% = 49%) are still evaluating the approaches and waiting for simplification (read “standardization”) of the procedure and more guidance from regulatory authorities. In other words: this innovation is currently situated in the “adoption chasm”:
Nonetheless, why is it important to start already now?
- ICH GCP changes, which comes into force in November 2016.
- Published EMA’s guidance for Risk Management Plan.
- Clear advantages of RBM compared to traditional monitoring
- Natural development of the pharma industry from “data capturing” to “intelligent cloud”
Summing up, those companies, which start today, capture the deepest experience and can later offer their expertise on the market for the majority of conservative parties and skeptics.







Leave A Comment
You must be logged in to post a comment.