Enhancing Clinical Trial Efficiency with API Integration in the MyRBQM Portal
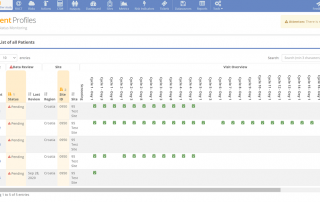
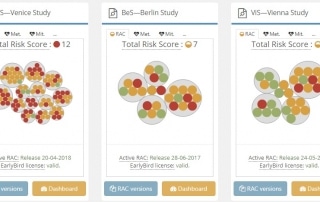
In the ever-evolving landscape of clinical trial risk management, effective communication between various systems is crucial for streamlined operations and regulatory compliance. The MyRBQM Portal, a leading risk-based quality management (RBQM) tool, introduces advanced API integration capabilities tailored to clinical operations and risk management [...]